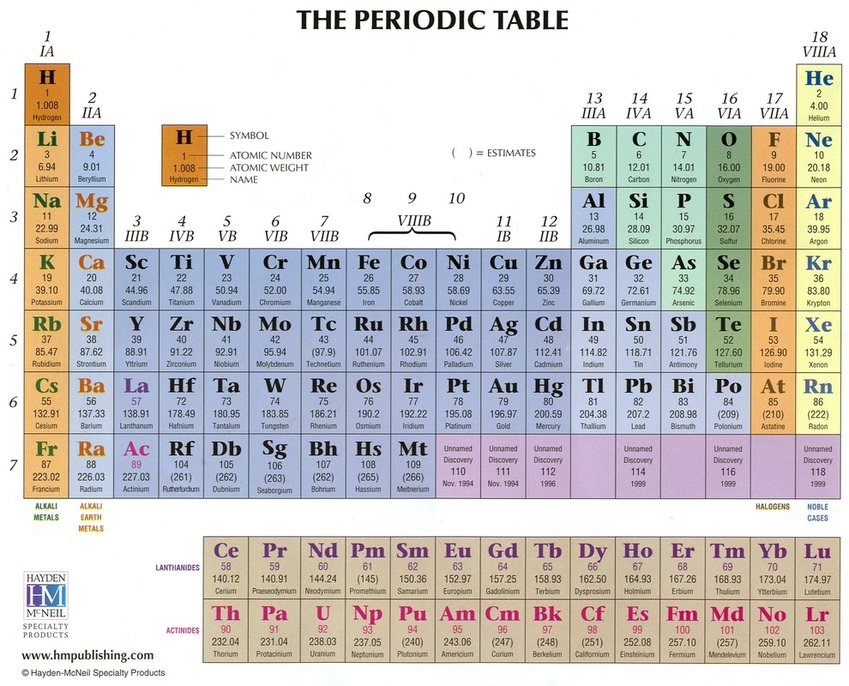
An element is a substance which cannot be broken down into simpler substances by chemical or physical means.
Elements are made up of atoms, which are the smallest particles of matter. Atoms contain protons, neutrons and electrons. Protons are positively charged, electrons are negatively charged, and neutrons, which are neutral, have no electric charge.
If two or more elements are chemically combines, they form a compound. Atoms can combine with one of the three following bonds: Covalent, ionic, or metallic. A covalent bond occurs when one atom shares electrons with another atom. An ionic bond occurs when a positive ion is attracted to a negative ion. A metallic bond is formed when one metal ion shares electrons with another metal ion.
Here is some vocabulary that you may need to know about atoms and the periodic table:
Elements are made up of atoms, which are the smallest particles of matter. Atoms contain protons, neutrons and electrons. Protons are positively charged, electrons are negatively charged, and neutrons, which are neutral, have no electric charge.
If two or more elements are chemically combines, they form a compound. Atoms can combine with one of the three following bonds: Covalent, ionic, or metallic. A covalent bond occurs when one atom shares electrons with another atom. An ionic bond occurs when a positive ion is attracted to a negative ion. A metallic bond is formed when one metal ion shares electrons with another metal ion.
Here is some vocabulary that you may need to know about atoms and the periodic table:
- Nucleus- theN very small, center core of an atom
- Proton- Thr particle of an atom with a positive charge
- Neutron- The part of an atom that is neutral
- Electron- The particle of an atom that moves rapidly in the space outside the neucleus; Has a negative charge
- Atomic Number- The number of protons in the nucleus of every atom of an element
- Mass Number- The sum of protons and neutrons in the nucleus of an atom
- Isotope- Atoms of the same ekement that differ in the number of neutrons, but have the same number of protons
- Model- An object that helps explain ideas about the natural world
^^^ This is a model known as the periodic table. Scientists organize element within the table by their properties. Columns in the periodic table are known as groups.